Anodizing is the process of creating a corrosion and damage resistant layer on metal surfaces by depositing an oxide layer on the surface of the metal. This process is especially effective for components with complicated structures. Additives can also be introduced to the anodizing process to help achieve desired characteristics.
CCI works closely with Aerospace customers to support their process with anodizing chemistry. CCI can offer a range of chemicals and chemical grades to support an anodizing operation. CCI offers raw materials for use in the different anodizing bath stages or can offer ready to use baths. Ready to use solutions for anodizing baths provide the advantage that critical parameters are guaranteed to be more consistent. Raw materials can also be offered in tandem to replenish the critical parameters to extend the baths life span. CCI offers anodizing chemistry and process chemicals in several package sizes to suit our customer’s needs, from totes to bottles.
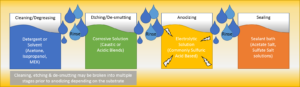
Anodizing Chemistry Stages
Anodizing consists of several steps, rinsing with DI Water between stages.
The first step is a general cleaning and degreasing of the surface of the substrate. This can be done with a detergent and water or with a solvent. This will allow the surfaces of the component to be exposed to the second step. Typical solvents used in this step include:
- Acetone
- Isopropyl Alcohol
- Methyl Ethyl Ketone
The second step is to remove the substrate’s natural oxides and imperfections with an etching solution. This etchant is typically an alkaline or acidic corrosive solution that specifically targets the substrate. Etchants are formulated to have specific etch rates at defined temperatures, dependent on the concentration and type of corrosives present. Some operations will employ an additional cleaning or de-smutting bath to ensure that all the unwanted particles are removed from the surface before anodizing. Typical corrosives used in these stages include:
- Hydrochloric Acid & HCL dilutions
- Nitric Acid & Nitric dilutions
- Sulfuric Acid & Sulfuric dilutions
- Phosphoric Acid & Phosphoric dilutions
- Hydrofluoric Acid & HF dilutions
- Sodium Hydroxide & NaOH dilutions
The Electrolytic Bath
Once the substrate is cleaned and all oxide films and imperfections are removed, it is ready to be anodized. The electrolyte in the anodizing step is composed of a corrosive dilution (sulfuric acid is commonly used) and any additives that are specific to the application.
A negative charge is passed thru the cathode, and the parts/components that are being anodized are given a positive charge, the anode. The resulting oxide layer characteristics are dependent on:
- Electrolyte parameters
- Electrical current being used (Amps)
- Temperature
- Anodizing time
- Substrate Material
After the anodizing step, a color bath can be used to apply a color if wanted.
The final step of the anodizing process is to seal the now porous oxide layer on the substrate. This will fill the gaps on the porous oxide layer and complete the process of making the components more resistant to corrosion and damage. This can be done chemically or thermally depending on the substrate. Chemical sealing is typically performed with metal salt baths formulated with:
- Acetate salts, such as Nickel Acetate
- Sulfate salts, such as Nickel Sulfate
Contact CCI to discuss your anodizing chemistry needs